Abstract
Background: Two prospective phase II studies evaluated the efficacy of the anti-CD38 monoclonal antibody Daratumumab as a single agent (sDARA) in patients with relapsed AL amyloidosis and reported impressive hematologic and organ response rates. However, both studies included small number of patients and in one study sDARA was administered for 6 cycles (n=40) while in the other study, it was administered until hematologic progression, toxicity or for up to 24 months (n=22). Furthermore, predictive factors associated with hematologic and organ responses, and optimal duration of therapy remain unclear.
Methods: We retrospectively studied the clinical and biological characteristics of patients with AL amyloidosis treated with sDARA, between April 2017 and December 2020, to identify factors associated with hematologic and organ response rates and impact on progression-free survival and overall survival (OS). Criteria for hematologic and organ response were defined according to the consensus criteria of ISA. Major organ deterioration progression-free survival (MOD-PFS) was used as a composite of endpoints from the time between sDARA initiation and whichever of the following occurred first: death, development of end stage cardiac or renal failure, or hematologic progression. OS was defined as the length of time between initiation of sDARA and the date of death or last follow-up. To account for immortal time bias, a landmark analysis for MOD-PFS and OS starting at the 12-month mark was performed to evaluate the impact of treatment duration on outcomes.
Results: Eighty six patients with AL amyloidosis received sDARA. The median age was 67 years (range, 39-86) and 65% were male, 75% with lambda AL amyloidosis. Of the 86 patients, 38% had t(11;14) and 11% had 1q gain. At time of sDARA initiation, 35% of patients had >2 organs involved, including 22% with BU stage III (14% IIIa and 8% IIIb) and 13% with renal stage III disease. Majority (44%) of the patients received sDara as 2 nd line therapy, but 2% received as frontline, 21% as 3 rd line and 33% as 4 th line or more. Half of the patients (45%) were previously treated with HDM/SCT and 87% with proteasome inhibitor based regimen. Patients received a median of 12 cycles of sDARA (range 1-36) with a median follow-up time of 21.6 months (range 1.2-48.3).
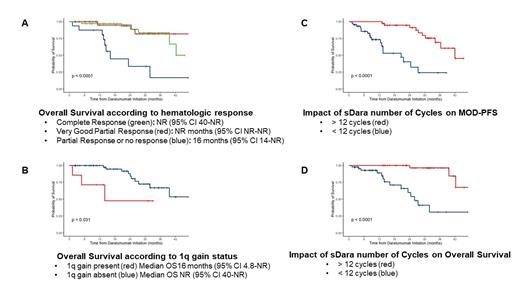
Hematologic CR and VGPR were achieved by 44% and 37% of patients, respectively and significantly associated with prolonged MOD-PFS and OS (Figure A and B). The median time to cardiac and renal responses were 6.8 months (range 0.9-12) and 6.7 months (range 1.8-42), respectively. At 12 months, cardiac and renal responses were observed in 47% and 61%, respectively and correlated with depth of hematologic response. The median OS and MOD-PFS were not reached (95% CI 40-NR) and 36 months (95% CI 28-NR), respectively. Achievement of cardiac response was associated with improved MOD-PFS (42 vs. 25 months; HR 0.25, 95% CI 0.08-0.78; p=0.01) and OS (NR vs 26 months; HR 0.20, 95% CI 0.04-0.89; p=0.02) and achievement of renal response was associated with improved MOD-PFS (NR vs. 13 months; HR 0.06, 95% CI 0.02-0.20; p<0.0001) and OS (NR vs. 25 months; HR 0.19, 95% CI 0.05-0.65; p=0.004). Importantly, presence of t(11;14) and number of previous lines of therapy did not impact MOD-PFS and OS. On an univariate analysis, several variables including dFLC >180 mg/L, bone marrow infiltration >10%, 24h proteinuria >3.5 g and NTproBNP >8500 pg/mL were significantly associated with lower MOD-PFS and OS, but only achievement of VGPR or CR and presence of 1q Gain were independently associated with MOD-PFS and OS (Figure C and D) on a multivariate analysis. Finally, on a landmark analysis, patients who received >12 cycles vs <12 cycles had significantly longer MOD-PFS (30 vs. 13 months; HR 0.47, 95% CI 0.31-0.72; p=0.0018)) and OS (NR vs. 15 months; HR 0.09, 95% CI 0.03-0.37; p<0.0001).
Conclusion: sDARA confers very high rates of hematologic responses (81% of patients achieving >VGPR) in patients with relapsed AL amyloidosis and leads to prolonged OS and MOD-PFS, which is independent of the number of previous lines of treatment. Our data confirmed that achievement of hematologic response is a major predictor of OS and MOD-PFS in AL amyloidosis, and revealed that presence of 1q Gain is associated with lower response rate to sDARA. Longer duration of therapy (>12 cycles) with sDARA was associated with prolonged MOD-PFS and OS.
Sloan: Stemline: Honoraria; Abbvie: Honoraria; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Pharmacosmos: Membership on an entity's Board of Directors or advisory committees. Sanchorawala: Karyopharm: Research Funding; Pfizer: Honoraria; Regeneron: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Prothena: Membership on an entity's Board of Directors or advisory committees, Research Funding; Proclara: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Caleum: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sorrento: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal